The decades-old test for ninhydrin-positive substances in individual amino acid monographs was scrapped in 2013. But is the current approach satisfactory? Butterworth Laboratories takes a deep dive into the evolving landscape of amino acid testing.
The general challenge posed by the analysis of free amino acids is that many degrade under the high sample injector temperatures required for gas chromatography (GC) analysis. This challenge is further compounded by the fact that while high-performance liquid chromatography (HPLC) procedures can efficiently separate even very complex mixtures of amino acids, the resolved sample components do not absorb UV radiation. This rules out the visualisation and recording of the separation process results using standard UV detectors.
The solution employed by the European Pharmacopoeia (PhEur) historically was to use low-resolution thin layer chromatography (TLC) procedures that first separated the sample amino acids followed by treatment of the TLC plate with ninhydrin reagent, which reacted with the resolved components to give coloured derivatives which could be assessed by simple visual inspection.
From 2013, the TLC method has been being replaced by a post-column derivatisation HPLC procedure, which adds ninhydrin reagent and heat to the mobile phase containing resolved sample components as it exits the analytical column. As with the TLC procedure, this gives coloured derivatives, which can be assessed using UV detection. Thus, when the TLC or HPLC test is prescribed in PhEur monographs, it is called “Ninhydrin-positive substances” (NPS).
The differences and similarities between TLC and HPLC
Where TLC is prescribed as the ninhydrin-positive substances (NPS) test in individual amino acid monographs, they contain all of the instructions for preparing analytical solutions and mobile phase, TLC plate selection, and detail the entire analytical procedure, in line with the general TLC monograph (2.2.27). The only system suitability demonstration required is a substance monograph-specific, two-component resolution.
When HPLC is prescribed as the NPS test in individual amino acid monographs, these also contain all the instructions for preparing the required analytical solutions. All monographs have the same requirement for the resolution of leucine and isoleucine to demonstrate system suitability. Furthermore, universal HPLC system suitability requirements addressing sensitivity and peak symmetry stipulated in the Chromatographic Separation Techniques general monograph 2.2.46 must also be fulfilled. The amino acid monographs do not detail the chromatographic system or procedure to be followed but state that the analysis should conform with general monograph 2.2.56 Amino acid analysis, Method 1. However, this contains no definitive chromatographic system or conditions but only states that ion-exchange chromatography with post-column ninhydrin derivatisation should be used.
To address shortcomings of the TLC test, the HPLC methodology has progressively replaced the requirement for this procedure in individual revised amino acid monographs. Full details of the two tests, comparative specifications, and practical advantages of the HPLC test over TLC are discussed later.
The following table shows the amino acid monographs, which have been revised to include the 2.2.56 HPLC NPS procedure.
| Ph.Eur Monograph Title | Monograph No. | Current Version Date |
| Alanine | 0752 | 01/2017 |
| Arginine | 0806 | 01/2017 |
| Arginine hydrochloride | 0805 | 08/2019 |
| Aspartic Acid | 0797 | 01/2018 |
| Cysteine hydrochloride monohydrate | 0895 | 08/2019 |
| Cystine | 0998 | 01/2019 |
| Glycine | 0614 | 08/2020 |
| Histidine | 0911 | 01/2017 |
| Histidine hydrochloride monohydrate | 0910 | 08/2019 |
| Isoleucine | 0770 | 01/2017 |
| Leucine | 0771 | 01/2017 |
| Lysine acetate | 2114 | 07/2023 |
| Lysine hydrochloride | 0930 | 01/2017 |
| Magnesium aspartate dihydrate | 1445 | 08/2019 |
| Phenylalanine | 0782 | 01/2017 |
| Proline | 0785 | 01/2017 |
| Serine | 0788 | 01/2017 |
| Threonine | 1049 | 01/2017 |
| Tryptophan | 1272 | 01/2017 |
| Tyrosine | 1161 | 01/2017 |
| Valine | 0796 | 01/2017 |
“Interestingly, the substance monographs that have adopted HPLC still retain the TLC as one of the identification requirements and, therefore, are still relevant,” says Frank Judge, consultant chemist, chromatography, Butterworth Laboratories.
TLC analysis
The TLC procedures resolve the sample components on a thin 20cm square glass TLC plate coated with a thin film (c.200 – 250µm) of either silica gel or cellulose as the chromatographic stationary phase. The sample is dissolved in a solvent, and a 200-fold dilution of this sample solution is prepared as a reference standard. The standard solution, therefore, has a concentration of 0.5% relative to the sample solution. A mobile phase consisting of acetic acid plus water plus Butanol (20/20/60) is used with cellulose TLC plates. Either the same mobile phase or ammonium and propanol (30/70) is used with silica gel plates. Both mobile phases are acidic.
Since the TLC mobile phase is acidic, it results in the protonation of the NH2 functional group of primary amino acids by a hydrogen ion, resulting in a positively charged NH3+ group, as shown below.
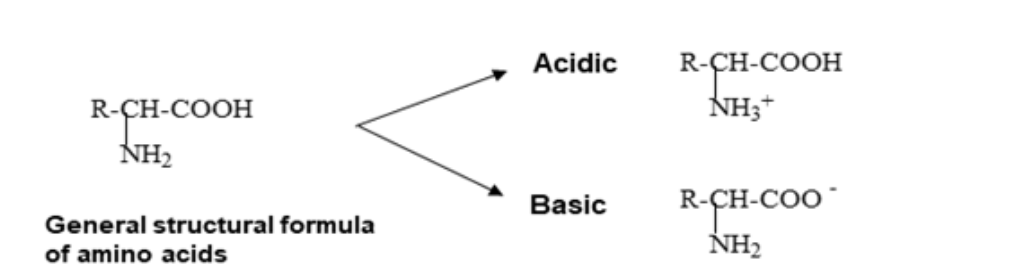
Whereas the NH functional group is protonated with secondary amines such as proline in acidic conditions to give a positively charged NH2+, as seen below.
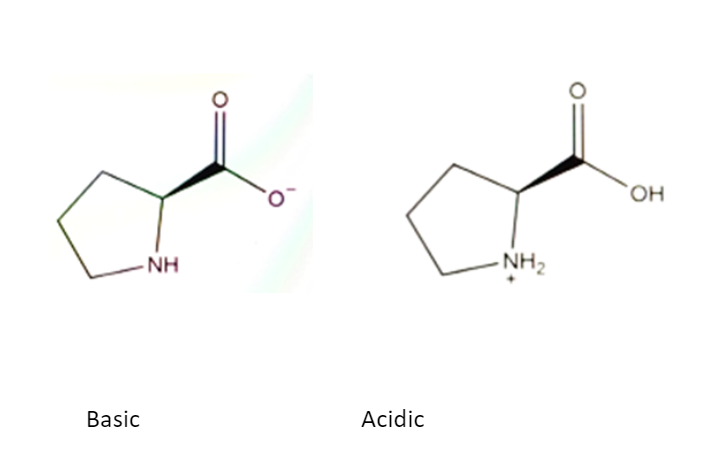
Source: Butterworth Labs
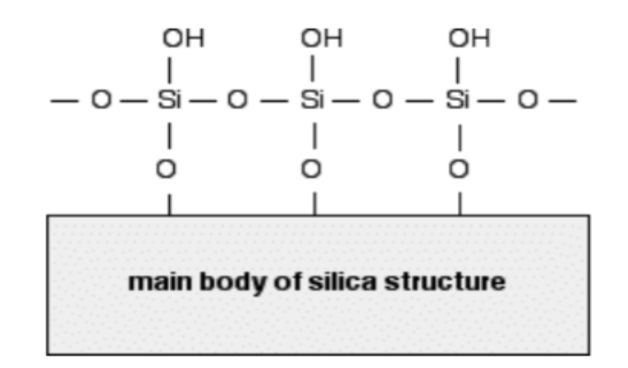
The structure of the silica gel plate’s stationary phase is illustrated below. Due to the electronegative oxygen atoms, the OH groups of the silica are partially negatively charged.
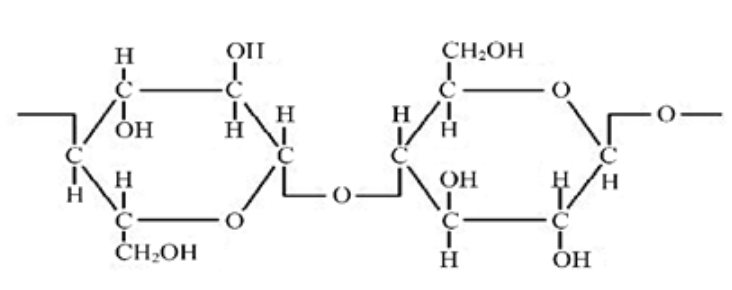
Similarly, with the structure of the cellulose stationary phase, the OH groups are partially negatively charged due to the electronegative oxygen atoms.
The positively charged NH3+ functional group of the protonated primary amino acids, or protonated NH2+ of secondary amino acids being carried up the TLC plate dissolved in the acidic mobile phase are attracted by the negative charge of the OH groups of the stationary phase, which slows the migration of the amino acids up the plate. The different R structures of specific amino acids affect the strength of, and therefore, the attraction of the positive charge of their NH3+ functional group to the negatively charged stationary phase of the TLC plate. This, along with their differing solubility in the mobile phase, results in specific amino acids travelling up the plate at different speeds resulting in the chromatographic separation.
After development, the TLC plate is dried in an oven, followed by applying the ninhydrin spray reagent and heat to the developed plate, which reacts with the separated amino acids, giving discreet coloured spots.
Most free amino acids contain a primary amine functional group. When derivatised with ninhydrin, this group gives compounds with a chromophore that absorbs electromagnetic radiation at 570nm, resulting in a violet-blue colour, as illustrated in the reaction mechanisms below.
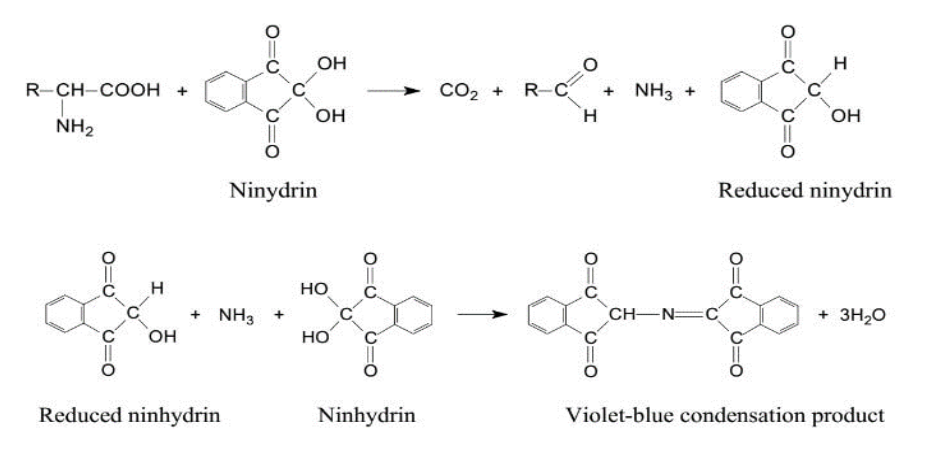
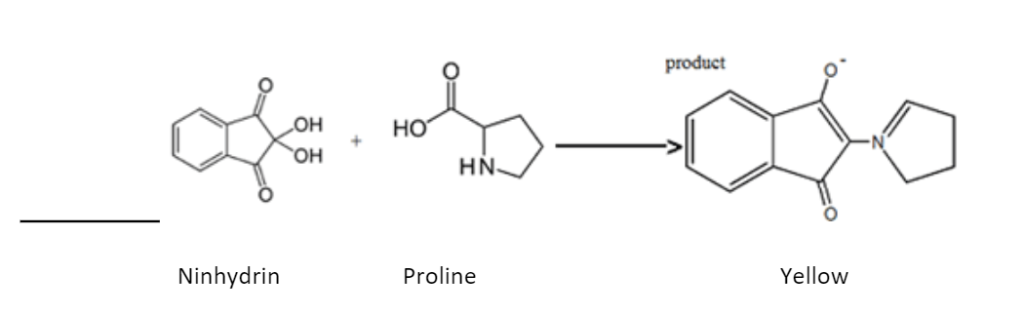
When derivatised with ninhydrin, amino acids with a secondary amine functional group, they produce products that absorb at 440nm and are yellow/orange.
HPLC analysis
The HPLC methodologies, adopted from 2013, are based on chromatographic separation by cation exchange with post-column ninhydrin derivatisation, resulting in the same coloured derivatives as with TLC, which are then amenable to dual-channel UV detection at 570 nm for primary amines and 440 nm for secondary amines. A lithium-based cation-exchange system gives the high resolution required for the analysis of physiological samples containing complex mixtures, including all classes of amino acids, and a lower resolution sodium-based system, which is adequately efficient and considerably faster for the analysis of the simple free amino acids included in PhEur monographs.
The HPLC columns used contain cation-exchange resin packing, generally in the form of small microbeads (0.25–1.43 mm radius) fabricated from an organic polymer substrate. The beads are typically porous (with a specific size distribution that will affect their properties), providing a large surface area on and inside them where the ion exchange process can take place. Most commercial resins are based on polystyrene sulfonate, as shown in the example below.
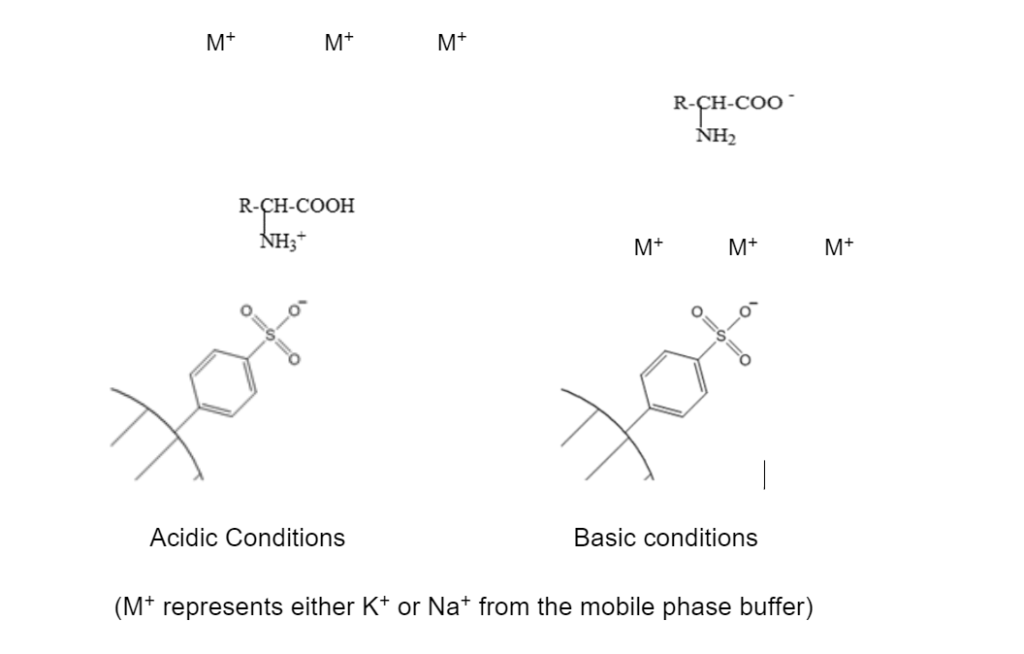
The aromatic sulfonate groups of the resin are strong enough acids that they are not protonated except under highly acidic pH values of < 1. Most of the analytical methods used for NPS analysis start with the mobile phase at pH 2 or 3, ensuring that the resin is negatively charged (SO3–). At this pH, the amino acid’s amine group (NH2) is protonated and positively charged (NH3+); thus, it will be attracted and bound to the negatively charged resin and will not progress through the HPLC column. As the analysis proceeds, the pH and ionic strength of the mobile phase buffer is progressively increased.
As the mobile phase becomes basic, the positively charged amino group is deprotonated, returning to the neutral state. At the same time, the carboxyl group (COOH) of the amino acid is also deprotonated and becomes negatively charged (COO–). This induces strong repulsion from the negatively charged resin, and the now negatively charged amino acid is released and replaced by M+ ions from the mobile phase as it progresses through the column. This replacement process is what gives the name ion-exchange. The exact nature of the R group of the different amino acids affects the precise pH and ionic strength at which ion exchange and release into the mobile phase will occur for each, which results in chromatographic resolution.
With secondary amines, the process is similar, with the protonation of their NH group to NH 2 + being responsible for attraction to the resin. At the end of each sample analysis, the column is washed by pumping a highly basic mobile phase solution of sodium hydroxide. This ensures that the column is stripped of all sample-related components in a process called regeneration. A relative 0.2% standard is prepared to analyse primary amine samples by diluting the sample solution. This standard is used to quantify other primary amine sample contaminants seen in the 570 nm UV detector channel. Secondary amine sample contaminants are quantified using an equivalent 0.2% standard of proline and responses seen in the 440 nm detector channel.
For analysis of secondary amine samples, a 0.2% standard of aniline is used for quantitation of primary amine contaminants using the 570 nm signal and a 0.2% dilution of the sample solution and the 440 nm detector channel is used for quantitation of secondary amine contaminants.
Below is a schematic showing a typical commercially available dedicated post-column ninhydrin HPLC amino acid analyser (AAA).
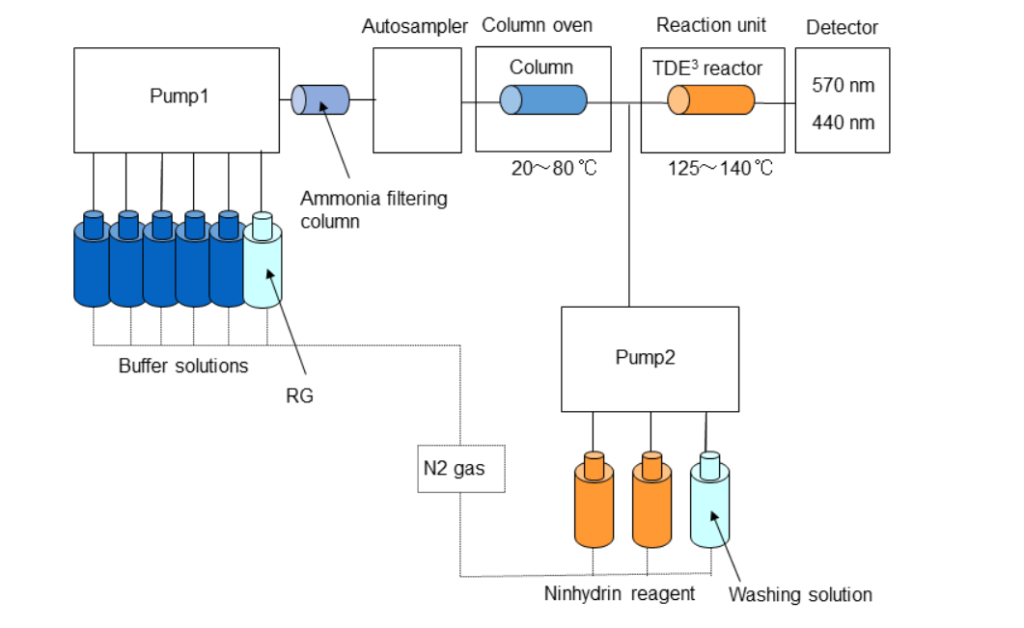
AAAs employ a mobile phase gradient proportioned from two to five individual buffer solutions depending on the specific system. The ninhydrin reagent is either a single solution that requires special storage conditions and has a short shelf-life or two solutions that are proportioned and mixed in real-time as needed by the system.
Below is a typical mixed primary amine standard chromatogram produced at Butterworth Laboratories Limited obtained with an AAA using five buffers and a two-part ninhydrin reagent conforming to the diagram above.
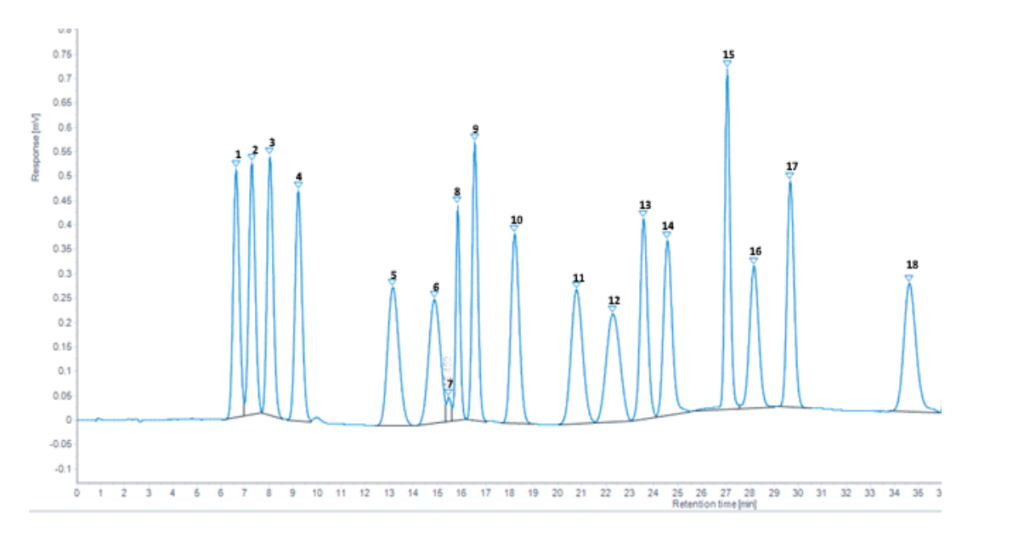
| Peak No. | Peak Identity | Peak No. | Peak Identity | Peak No. | Peak Identity |
| 1 | Aspartic Acid | 7 | Unknown | 13 | Tyrosine |
| 2 | Threonine | 8 | Cystine | 14 | Phenylalanine |
| 3 | Serine | 9 | Valine | 15 | Lysine |
| 4 | Glutamic Acid | 10 | Methionine | 16 | Ammonium |
| 5 | Glycine | 11 | iso-Lucine | 17 | Histidine |
| 6 | Alanine | 12 | Lucine | 18 | Arginine |
Source: Butterworth Labs
Conclusion
“In pharmacopoeial terms, the revision of individual amino acid monographs to replace the TLC NPS test with HPLC was carried out relatively quickly for the most relevant pharmaceutical raw materials once started,” says Judge. “Although the general monograph 2.2.56, published in 2004, was not prescribed for use by an amino acid monograph until 2013, between 2013 and 2017, 13 monographs were revised and, as at the time of producing this article, the number is 21.
“My thoughts are that the first two monographs to be revised were for leucine and isoleucine because these amino acids are difficult to resolve even by HPLC, and this resolution has subsequently been required as a demonstration of system suitability in all primary amine monographs that include the HPLC procedure.
“The PhEur amino acid monographs that have adopted HPLC to determine ninhydrin-positive substances still retain the TLC as one of the identification requirements and, therefore, are still relevant, which may be surprising. I think the TLC procedure remains to allow continued confirmation of identity using a simple test without the requirement for highly specialist AAA instrumentation. This facilitates inexpensive identity testing to be applied to individual containers of every batch of raw material used in production while using the HPLC method for testing fewer composite samples at the same time.”
Butterworth scientists see the fundamental challenges with the current TLC procedure as follows:
- It does not resolve all amino acids and is not particularly sensitive.
- It is subjective since the determination of impurities is based on the visual estimation of the relative intensities of standard and sample impurity spots.
- Most of the monographs are for primary amines, and the standard is a dilution of the sample preparation; if any such samples contain secondary amino acid contaminants, then the comparison of relative intensities is not meaningful since the colours of the derivatised primary and secondary amines are very different.
- As the specification for the test states that there should be no individual impurity spots from the sample exceeding the intensity of the relative 0.5% standard, and no sum impurity determination can be obtained, samples could contain several different impurities, which individually meet the specification. However, they would not be considered compliant if they could be totalled.
They see the advantages of the HPLC procedure as follows:
- The analytical process is automated, and it fully resolves the majority of amino acids.
- Since UV detection replaces visual comparison, HPLC is more sensitive, and precise determination of the relative responses of standard and sample components allows quantitation of individual and total impurities.
- Since primary and secondary amino acid sample contaminants are determined using primary and secondary standards with little interference due to the use of discreet UV channels for each, results are genuinely semi-quantitative.
- The procedure applies a limit of 0.1% for individual impurities and a sum limit of 1.0%.
Reports that ninhydrin derivatisation is maintained in the HPLC procedures to give results comparable with those of TLC are not the case, says Judge, who concludes that it might have been better to replace TLC with a manual pre-column derivatisation procedure which could be accomplished using standard HPLC equipment rather than expensive and specialised AAA.


