If you would like to be kept up to date with our latest blogs, join our mailing list.
We value your privacy, so we never share our mailing list with third parties.
For over 40 years, Butterworth Laboratories has provided independent, contract analytical services to the global pharmaceutical and related industries.
If you would like to be kept up to date with our latest blogs, join our mailing list.
We value your privacy, so we never share our mailing list with third parties.
Posted on Friday July 4, 2025
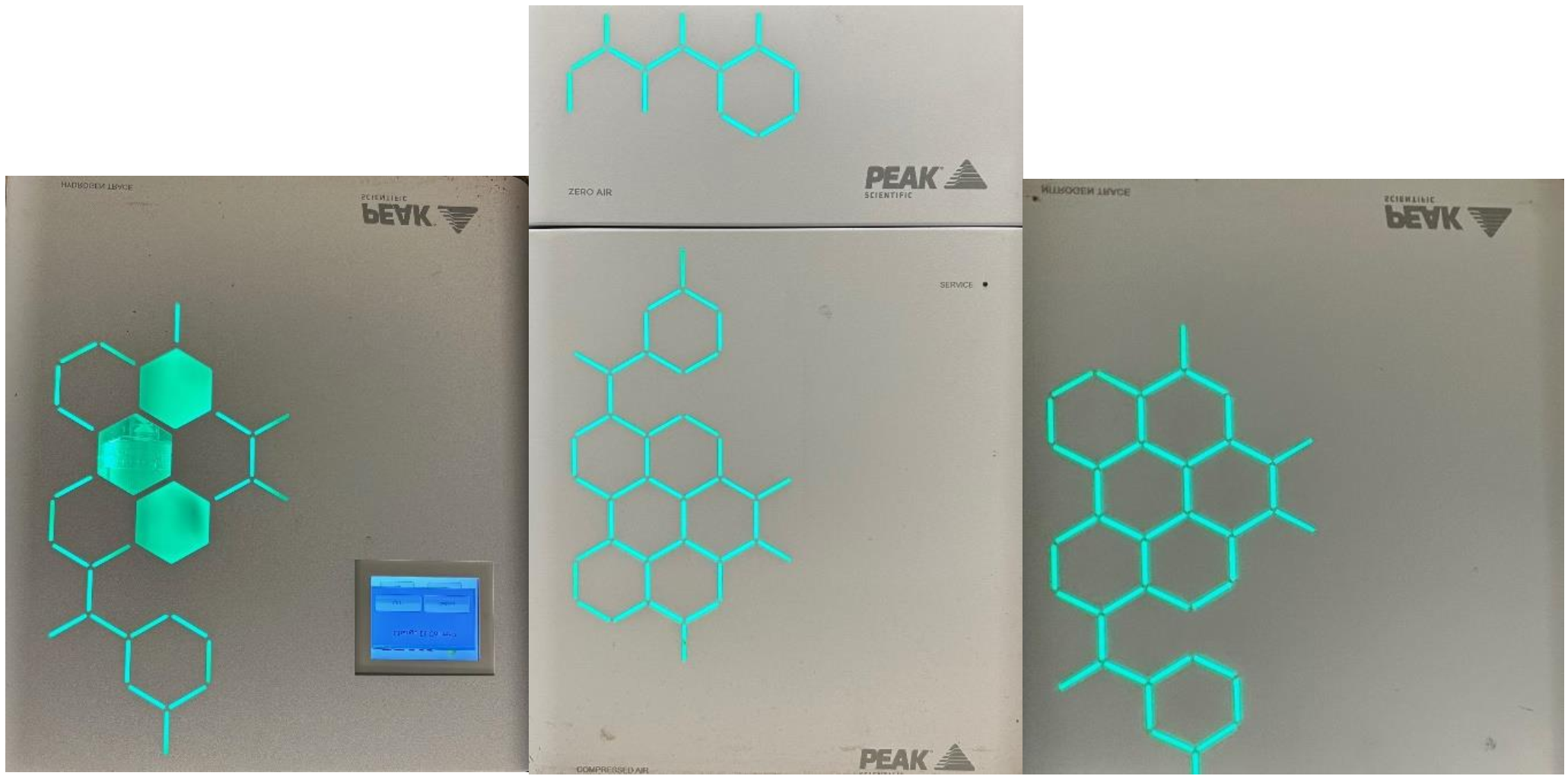
About a year ago, Butterworth Laboratories acquired three new Gas Generators. Our laboratory managers had long considered this decision, which was driven by the numerous advantages gas generators offer over traditional gas cylinders. Below is a summary of our experience with these new systems so far. Precision Zero Air Generator The Precision Zero Air generator […]
Read More
Posted on Wednesday May 14, 2025
The fourth period of global helium shortages (HS 4.0) in the last 16 years ended during Q1 to Q2 of 2024, when the market finally returned to a plentiful market supply. Unsettling publications regarding general supply issues focus on the finite nature of helium and the nightmare backdrop of ‘running out’. However, none of these […]
Read More
Posted on Friday April 25, 2025
At our analytical laboratory, we operate with robust safety protocols, risk assessments, and engineering controls to protect our staff and visitors from harm. However, even the most reliable control measures are not infallible. That’s why being prepared for first aid emergencies is not just a legal obligation it’s a critical component of a resilient safety […]
Read More
Posted on Thursday April 17, 2025
As discussed in my previous blog, HS 4.0 and the three earlier periods of supply disruption and associated price increases over the past 16 years were not linked to global reserves or resources. Shortages have arisen from industrial accidents, fires that destroyed extraction plants, unplanned maintenance, geopolitical events, and poor US Federal Helium Reserve management. […]
Read More
Posted on Wednesday April 9, 2025
With preparations at Butterworth Laboratories well underway for the Making Pharma 2025 conference in April, I thought an update might be interesting. It is a follow-up to my own helium shortage presentation at the conference in 2024, which appeared to be well received. After 16 years of continued periodic shortages, 2022 had been expected to […]
Read More
Posted on Tuesday March 11, 2025
At Butterworth Laboratories, we recognise that our people are our greatest asset. A healthy and supportive workplace starts with looking after both our own wellbeing and that of our colleagues. That’s why, for My Whole Self Day 2025, we are introducing the My Whole Self MOT, a simple yet effective way to check in on your own mental […]
Read More
Posted on Tuesday December 17, 2024
Since e-cigarette and vape devices were introduced, their popularity has sky-rocketed. Initially brought in as a ‘diet’ version of regular tobacco smoking, with emphasis on being a way to wean smokers off their habit, vaping has grown ever more prevalent, particularly amongst the younger population. Vape liquids contain nicotine and flavourings with a mixture of […]
Read More
Posted on Thursday December 12, 2024
As Western pharmaceutical companies increasingly target the Chinese market, there is a growing demand for raw materials testing in accordance with the Chinese Pharmacopeia (ChP). At Butterworth Laboratories, we possess extensive experience with this text and are well-acquainted with its nuances. The current edition, the 11th, was released in July 2020. However, the official English […]
Read More
Posted on Tuesday December 10, 2024
As part of our occasional series of articles explaining some of the key analytical techniques we use at Butterworth, we thought it would be useful to provide some background information on one of our main instruments used for metals analysis. Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) is a technique used to determine trace elements in […]
Read More
Posted on Wednesday August 21, 2024
Take, for example, the average Butterworth Laboratories Ltd in-house method for chromatographic impurities, which is typically around 7 pages long and has over 2000 words. The method will include important sections like Quality Control to ensure that it continues to operate within its validated parameters and specific instrument parameters that have been validated on the […]
Read More
Posted on Thursday August 8, 2024
In part one of our viscous Newtonian explorations, I examined kinematic or capillary viscosity measurement and briefly discussed a Newtonian liquid. In this part, we will examine sample preparation and rotational or apparent viscosity. One of the main causes of Out-of-Specification results when testing solid samples is sample preparation issues. These can be further divided […]
Read More
Posted on Friday August 2, 2024
Viscosity determination can cause many problems, mainly due to misunderstandings of the various techniques, such as measurement conditions, settings and units. Let’s see if we can shed some light on the subject. The pharmaceutical, consumer healthcare and cosmetic industries frequently use excipients to ensure their products have the right thickness to ensure correct flow through […]
Read More
Posted on Wednesday July 31, 2024
Karl Fischer’s (KF) water determination is a vital and well-established method, but it can be fraught with difficulties and complexities. A material’s water content is often used to correct assays or other tests to give results on an anhydrous basis. So, no matter how good the assay itself is, the whole analysis may be compromised […]
Read More
Posted on Wednesday May 1, 2024
As an old chromatography guy, I am certainly no helium expert; I always understood the ‘Helium Crisis’ from only a GC perspective. I found useful information published in our favourite chromatography magazines and many articles from analytical column, GC and gas generator manufacturers discussing technical aspects of substitution with hydrogen. I knew that Helium was […]
Read More
Posted on Tuesday April 30, 2024
For decades, Butterworth has been using hydriodic acid in the form of the Zeisel reaction to strip themethoxy, ethoxy and hydroxypropoxy groups off various cellulose backbones. The assay ofhypromellose, methyl-, ethyl- and hydroxypropyl cellulose type samples to the Ph.Eur., USP, JP andChP involves a complex and hazardous reaction via the adipic acid catalysed cleavage of […]
Read More
Posted on Monday March 25, 2024
1996 saw the implementation of the first automated headspace GC system in response to increased client requirements. Much of this testing in accordance with USP <467> Organic Volatile Impurities, however, was “blind compliance” since the 7 targeted solvents, including Benzene, were practically never used in the production of pharmaceutical articles and other solvents known to […]
Read More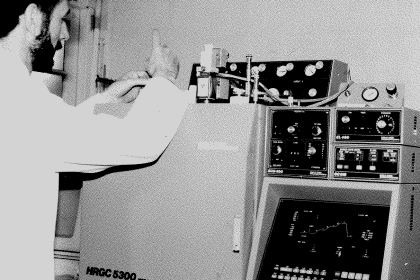
Posted on Tuesday March 12, 2024
I burnt my fingers this morning, removing my breakfast from a jammed toaster. After many years of gas chromatography, I am not unfamiliar with the occasional blister. When I arrived at the lab, someone shared a copy of a 30-year-old photo of David Bell, who made the most significant personal contribution to the development of […]
Read More
Posted on Thursday March 7, 2024
Having arrived in the chromatography laboratory at Butterworth’s in September 1994, the first work I was assigned was the determination of the purity of fire suppressant gases. The analysis was to be performed using a Carlo Erba HRGC 5300 GC equipped with a gas sampling valve. Over the next 15 years, I would develop a […]
Read More
Posted on Tuesday February 27, 2024
Have we stopped appreciating this chromatographic technique? Perhaps we have, especially those under the age of 40. With the advent of computerised chromatography data systems (CDS) controlling advanced pieces of instrumentation, GC, HPLC, IC, et al., there does appear to be a somewhat unfair dismissal of TLC as the aforementioned chromatography techniques’ inferior sibling. Those […]
Read More
Posted on Thursday December 21, 2023
It was 1991, and I had arrived at Berridge Environmental Laboratories in Chelmsford, hot off the train from Edinburgh, where I had just completed a 5-year joint honours degree. As shared in previous Blogs, I was being mentored by Dennis, a semi-retired elderly gentleman with a shock of white hair and asbestos fingers from all […]
Read More
Posted on Thursday November 16, 2023
It was 1991 and I had arrived at Berridge Environmental Laboratories in Chelmsford, hot off the train from Edinburgh where I had just completed a 5-year joint honours degree. As shared in previous Blogs, I was being mentored by Dennis, a semi-retired elderly gentleman with a shock of white hair and asbestos fingers from all […]
Read More
Posted on Thursday November 2, 2023
In our April News Bulletin, we highlighted an Article and White Paper written by our Associate Director, John Welch on the importance of the testing of excipients used in the manufacture of medicines. In both of these publications, John referenced the example of cough syrup which was manufactured by an Indonesian company between 2021 and […]
Read More
Posted on Wednesday October 18, 2023
Are we on the cusp of a helium crisis? There are many examples of scientists predicting that global helium reserves will be depleted in 20 to 35 years but we don’t believe this is credible. The Mineral Commodities Summaries 2023, produced by the US Geological Survey, part of the US Department of the Interior, reports […]
Read More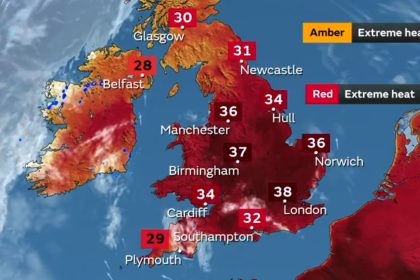
Posted on Friday June 23, 2023
Ambient temperatures in the UK are not something we usually associate as a potential risk factor. But as a weather map from the summer of 2022 illustrates, is this something we should be taking more notice of? Butterworth is used to receiving samples from clients in packaging containing temperature monitors for samples that must be […]
Read More
Posted on Wednesday June 14, 2023
It was 1991 and I had arrived at Berridge Environmental Laboratories in Chelmsford, hot off the train from Edinburgh where I had just completed a 5-year joint honours degree. As shared in previous Blogs, I was being mentored by Dennis, a semi-retired elderly gentleman with a shock of white hair and asbestos fingers from all […]
Read More
Posted on Friday June 9, 2023
At the start of May 2023, the FDA published a new Guidance for Industry document; Testing of Glycerin, Propylene Glycol, Maltitol Solution, Hydrogenated Starch Hydrolysate, Sorbitol Solution, and other High-Risk Drug Components for Diethylene Glycol (DEG) and Ethylene Glycol (EG). My own research on this subject has been very limited, however, three things are clear. […]
Read More
Posted on Thursday February 23, 2023
The answer is NO if the chips are mounted on a printed circuit board (PCB). Manufacturers of PCBs have long understood the correlation between surface cleanliness and corrosion, electrochemical migration, dendritic growth and the resultant, current leakage, and shorting. This means that clean room technology utilised by the industry is very stringent and far exceeds […]
Read More
Posted on Thursday February 2, 2023
It was 1991 and I had arrived at Berridge Environmental Laboratories in Chelmsford, hot off the train from Edinburgh where I had just completed a 5-year joint honours degree. As shared in my last Blog, I was being mentored by Dennis, a semi-retired elderly gentleman with a shock of white hair and asbestos fingers from […]
Read More
Posted on Friday January 13, 2023
Christmas came early this year for chromatographers. After a 30 year career-long wait, on 01/12/2022, the USP General Chapter <621>Chromatography, and the JP Chromatography General Chapter were finally fully harmonised with the Ph Eur. General Chapter 2.2.46 Chromatographic Separation Techniques. The good news includes that allowable adjustments of chromatographic conditions, which were especially complex for […]
Read More
Posted on Thursday November 17, 2022
It was 1991 and I had arrived for my first day at Berridge Environmental Laboratories in Chelmsford, hot off the train from Edinburgh where I had just completed a 5 year joint honours degree. This was to be the first day of my being paid as a chromatographer. I reported to the team leader of […]
Read More
The latest technology combined with our expert team of scientists make us leaders in Quality Control Testing, Method Development, Method Validation and Stability Testing of both pharmaceutical raw materials and finished products.
We refine this service by promoting a culture of open communication between our analysts and our clients, ensuring that you retain control of your work.
Request a quote Make an enquiryPrivacy Policy
Terms & Conditions
Sitemap
Registered in England and Wales
Company Registration No: 1185121
In accordance with The Data Protection Act 1998, we are registered with the Information Commissioner’s Office under registration reference Z3601936.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |