MAKING PHARMACEUTICALS 2025 REPORT

We’re pleased to report that this years Making Pharmaceuticals Exhibition was highly successful. According to the event organisers, the delegate attendance on the first day alone surpassed the total number of visitors for the event in 2024—a trend reflected in the volume of interest we received at our stand, with enquiries nearly doubling compared to last year. This year, most enquiries appeared to be focused on our Gas Chromatography services. We are now in discussions with a number of potential new clients.
Butterworth Laboratories was also represented in the seminar programme, with Jignasha Patel (QA Officer) and Elena Karayianni (Team Leader – Metals) attending various sessions. Both praised the high quality and relevance of the presentations.
Following the success of this years event, we’ve already secured our place for 2026. The 2026 edition promises to be even more impactful, with a new Nutraceuticals section.
NEW WEBSITE HOMEPAGE
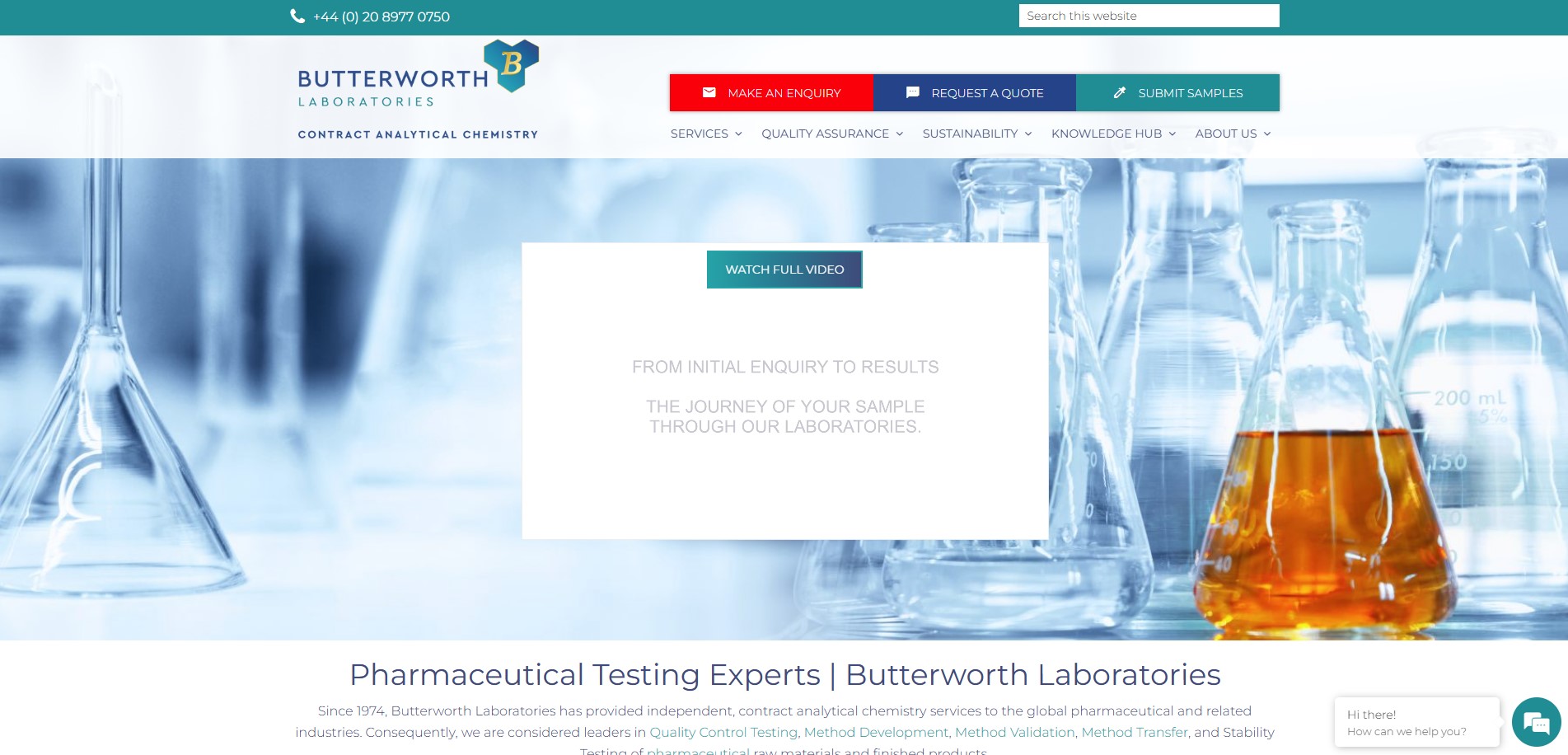
We have just launched a redesign of our website homepage. This has been done to enable visitors to find relevant information more easily. The menu bar has been rearranged to have separate entries for Quality Assurance and Sustainability. This should make locating our Quality Certificates easier. We have also added a Search function in the top bar and easy access to a video entitled ‘From Initial Enquiry to Results – The Journey of Your Sample Through Our Laboratories’.
To view our Home Page, click here.
LC-MS UPDATE

We are pleased to announce that our new Agilent LC-MS/MS has been commissioned and has already been put to good use with the successful completion of a development and validation study for the determination of alkaloids in an API. Further updates will be provided in future news bulletins.
CHEMUK 2025

Mala Patel and John Welch attended this event at the NEC. It was well attended and they had the opportunity to speak with a number of chemical manufacturers and suppliers. There was a strong emphasis on Sustainability throughout the event and a number of ideas were gathered for future discussion at Butterworth.